Abstract
Background: The majority of patients with myelodysplastic syndromes (MDS) needs regular blood transfusions and becomes transfusion dependent. Blood transfusions temporarily alleviate anemia-related symptoms, but can be accompanied by adverse events, like iron overload. In MDS patients with >20 blood transfusions and plasma ferritin levels >1000 µg/L, (inter)national guidelines advise iron chelation therapy (ICT) to reduce potential organ damage due to transfusion-mediated iron overload. The aim of this study was to determine the adherence to these guidelines, to determine to what extent plasma ferritin levels were monitored in daily practice and which patient-related factors contributed to monitoring plasma ferritin levels during the transfusion period.
Methods: We performed an observational, retrospective, population-based study, using the Hemobase registry, in MDS patients diagnosed between 2005 and 2017. The Hemobase registry is a population-based registry for all hemato-oncological patients diagnosed since 2005 in Friesland, a province of the Netherlands with approximately 650.000 inhabitants. It provides detailed clinical information on diagnosis and follow-up in daily clinical practice. Clinical information on blood transfusions, plasma ferritin measurements, treatment with ICT, and patient-related factors as age, comorbidities (according to the Charlson Comorbidity Index (CCI)) and revised international prognostic scoring system (IPSS-R) risk category was collected from electronic health records. Information about all distributed blood transfusions was available from the laboratory systems. RBC units were administered to patients according to and following national guidelines. Transfusion dependence was defined according to the International Working Group (IWG) 2018 guidelines: low transfusion burden (LTB) or high transfusion burden (HTB). LTB was defined as 3-7 transfusions in a period of 16 weeks; HTB was defined as ≥8 transfusions in a period of 16 weeks. MDS patients with International Prognostic Scoring System (IPSS-R) (very) low and intermediate were categorized as low-risk MDS, and patients with IPSS-R (very) high as high-risk MDS. Logistic regression analyses were performed to analyze the likelihood of monitoring plasma ferritin levels for patients of different subgroups: age >80 years vs. ≤80 y, CCI score ≥2 vs. <2, and IPSS-R high vs. low risk MDS.
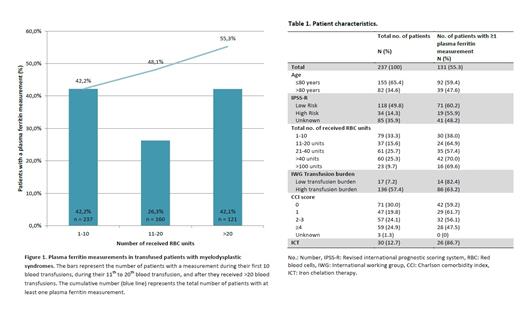
Results: A total of 237 out of 292 MDS patients (81.1%) received at least one transfusion. Plasma ferritin levels were measured at least once in 55.3% (n=131) of these patients (Table 1 and Figure 1). During the first 20 transfusions, plasma ferritin measurements were present in 114 of 237 patients (48.1%). After >20 transfusions, plasma ferritin measurements were present in 51 of 121 patients (42.1%). Age, comorbidities and IPSS-R score were not associated with the likelihood of monitoring plasma ferritin levels. 112 patients were eligible for ICT (i.e. plasma ferritin levels >1000 µg/L or >20 transfusions). ICT was given to 22.3% (n=25) of these patients. Five patients received ICT whilst not fulfilling the criteria according to the guideline. During ICT, plasma ferritin levels were monitored in 60.0% of patients with ICT.
Conclusions: In conclusion, in this population-based study, plasma ferritin levels were measured, irrespective of age, comorbidities and IPSS-R score, in 55% of MDS patients with transfusions. ICT was not given to all eligible patients and during ICT, monitoring of plasma ferritin levels was not performed in all cases.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal